SPAFMSR






Buzgar N., Apopei A. I., Buzatu A. (2009) - Romanian Database of Raman Spectroscopy (http://rdrs.uaic.ro)
| Why donate? If you use this site regularly and would like to help us, please consider donating a small sum. All proceeds go to: + development of this website; + buy some minerals not available yet; + development of RDSS software; + buy some articles or books necessary for Raman study (interpretation). |
Formula: |
SrSO4 |
|
Crystal Data: |
Crystal System: Orthorhombic - Dipyramidal |
|
Point Group: 2/m 2/m 2/m |
||
Cell Data: |
Space Group: Pbnm , a = 8.359, b = 5.352, c = 6.866, Z = 4 |
|
Density (calc.) = 3.97 and V = 307.17 Å3 |
||
| Element color: Sr, S, O | ||
 |
Sample no. 5515 from the "Mineralogy and Petrography Museum Grigore Cobălcescu" of "Alexandru Ioan Cuza" University, Iaşi. Origin: Gloucestershire - England. Click image to enlarge |
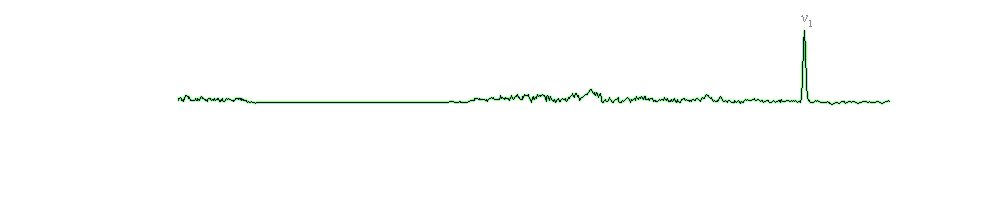
For celestine, Raman spectrum is also dominated (like barite and anglesite) by an intense ν1 band (1003 cm-1) and the other characteristic vibrational modes of SO4 group: ν2 - 461 cm-1; ν3 - 1111 cm-1, and 1160 cm-1; ν4 - 622 cm-1 and 641 cm-1. These values are similar to those reported in the literature.
| Celestine | Assignments | |
| Buzgar et al., 20091 | Kloprogge et al., 2001 | |
| 453 | ν2 SO4 | |
| 461 | 460 | |
| 622 | 620 | ν4 SO4 |
| 641 | 640 | |
| 659 | ||
| 1003 | 1003 | ν1 SO4 |
| 1096 | ν3 SO4 | |
| 1111 | 1112 | |
| 1160 | 1162 | |
The peak corresponding to the symmetric stretching mode (ν1) of the S-O bond in the sulfates appears to be the strongest in the Raman scattering intensity. The wavenumber of a Raman shift is determined by the bond strength and the atomic masses (Fadini and Schnepel, 1989). Therefore, the ν1 wavenumber is a function of S-O stretching force constant in sulfates and increases with an increase in the force constant. The force constant of SO4 tetrahedra were calculated from the bond lengths and the infrared absorption frequencies by Miyake et al. (1978).

Figure 1. Wavenumber of the ν1 vibrational Raman mode vs. atomic mass of the cations for barite group. |
The stretching force constants are: for barite K = 6,27 md/Å; for celestine K = 6.34 md/Å; and for anglesite K = 5.98 md/Å. Therefore, this systematic wavenumber shift is largely due to the increase of mean force constants because of substitution of larger cations in the M sites (Lee et al., 2005). Also, the wavenumber decreases with an increase of the atomic mass of the cations. An example for ν1 mode is presented in figure 1, where the atomic masses are: Sr = 87.62 u; Ba = 137.32 u; Pb = 207.2 u. |
• The Mineralogy Database [link]
• Crystal data (.cif file) from the American Mineralogist Crystal Structure Database [link]
• 1BUZGAR N., BUZATU A., SANISLAV I. V. (2009) - The Raman study on certain sulfates. Analele Stiintifice ale Universitatii “Al. I. Cuza” - Iasi, Tome 55, issue 1, 5-23 [link]
• Kloprogge, J. T., Schuiling, R. D., Ding, Z., Hickey, L., Wharton, D., Frost, R. L. (2002) - Vibrational spectroscopic study of syngenite formed during the treatment of liquid manure with sulphuric acid. Vibrational Spectroscopy, 28, 209-221. [link]
• Fadini, A., Schnepel, F. M. (1989) - Vibrational spectroscopy methods and applications. Ellis Horwood Limited.
• Miyake, M., Minato, I., Morikawa, H., Iwai, S. (1978) - Crystal structures and sulphate force constants of barite, celestite and anglesite. American Mineralogist, 63, 506–510. [link]